Навигация
4. Occurrence
According to theory, the stable isotopes 6Li and 7Li were created in the Big Bang, but the amounts are unclear. Lithium is a fusion fuel in main sequence stars. Because of the method by which elements are built up by fusion in stars, there is a general trend in the cosmos that the lighter elements are more common. However, lithium (element number 3) is tied with krypton as the 32nd/33rd most abundant element in the cosmos (see Cosmochemical Periodic Table of the Elements in the Solar System), being less common than any element between carbon (element 6) and scandium (element 21). It is not until atomic number 36 (krypton) and beyond that chemical elements are found to be universally less common in the cosmos than lithium. The reasons have to do with the failure of any good mechanisms to synthesize lithium in the fusion reactions between nuclides in supernovae. Due to the absence of any quasi-stable nuclide with five nucleons, nuclei of lithium-5 produced from helium and a proton has no time to fuse with a second proton or neutron to form a six nucleon isotope which might decay to lithium-6, even under extreme conditions of bombardment. Also, the product of helium-helium fusion (berylium-8) is immediately unstable toward disintegration to helium again, and is thus not available for formation of lithium. Some lithium-7 is formed in the pp III branch of the proton-proton chain in main sequence and red giant stars, but it is normally consumed by lithium burning as fast as it is formed. This leaves new formation of the stable isotopes lithium 6 and 7 to rare cosmic ray spallation on carbon or other elements in cosmic dust. Meanwhile, existing Li-6 and Li-7 is destroyed in many nuclear reactions in supernovae and by lithium burning in main sequence stars, resulting in net removal of lithium from the cosmos. In turn the destruction of lithium isotopes is due to their very low energy of binding per nucleon with regard to all other nuclides save deuterium (also destroyed in stars) and helium-3.[5] This low energy of binding encourages breakup of lithium in favor of more tightly-bound nuclides under thermonuclear reaction conditions.
Lithium is widely distributed on Earth but does not naturally occur in elemental form due to its high reactivity.[8] Estimates for crustal content range from 20 to 70 ppm by weight.[12] In keeping with its name, lithium forms a minor part of igneous rocks, with the largest concentrations in granites. Granitic pegmatites also provide the greatest abundance of lithium-containing minerals, with spodumene and petalite being the most commercially viable sources.[12] A newer source for lithium is hectorite clay, the only active development of which is through the Western Lithium Corporation in the United States.[34]
According to the Handbook of Lithium and Natural Calcium, "Lithium is a comparatively rare element, although it is found in many rocks and some brines, but always in very low concentrations. There are a fairly large number of both lithium mineral and brine deposits but only comparatively a few of them are of actual or potential commercial value. Many are very small, others are too low in grade."[35] At 20 mg lithium per kg of Earth's crust [36], lithium is the 25th most abundant element. Nickel and lead have the about the same abundance.
The largest reserve base of lithium is in the Salar de Uyuni area of Bolivia, which has 5.4 million tons. According to the US Geological Survey, the production and reserves of lithium in metric tons are as follows[37]:
Contrary to the USGS data in the table, other estimates put Chile's reserve base at 7,520,000 metric tons of lithium, and Argentina's at 6,000,000 metric tons.[38] Seawater contains an estimated 230 billion tons of lithium, though at a low concentration of 0.1 to 0.2 ppm.
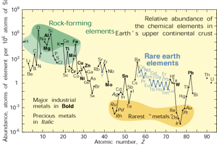
Figure. 2. Lithium is about as common as chlorine in the Earth's upper continental crust, on a per-atom basis.
5. Production
Since the end of World War II lithium metal production has greatly increased. The metal is separated from other elements in igneous minerals such as those above. Lithium salts are extracted from the water of mineral springs, brine pools and brine deposits.
The metal is produced electrolytically from a mixture of fused lithium and potassium chloride. In 1998 it was about US$ 43 per pound ($95 per kg).[40]
Deposits of lithium are found in South America throughout the Andes mountain chain. Chile is the leading lithium metal producer, followed by Argentina. Both countries recover the lithium from brine pools. In the United States lithium is recovered from brine pools in Nevada.[41] Nearly half the world's known reserves are located in Bolivia, a nation sitting along the central eastern slope of the Andes. In 2009 Bolivia is negotiating with Japanese, French, and even Korean firms to begin extraction.[42] According to the US Geological Survey, Bolivia's Uyuni Desert has 5.4 million tons of lithium, which can be used to make batteries for hybrid and electric vehicles.[42][43]
China may emerge as a significant producer of brine-source lithium carbonate around 2010. There is potential production of up to 55,000 tons per year if projects in Qinghai province and Tibet proceed.[44]
The total amount of lithium recoverable from global reserves has been estimated at 35 million tonnes, which includes 15 million tons of the known global lithium reserve base.[45]
In 1976 a National Research Council Panel estimated lithium resources at 10.6 million tons for the Western World.[46] With the inclusion of Russian and Chinese resources as well as new discoveries in Australia, Serbia, Argentina and the United States, the total had nearly tripled by 2008.[47][48]

Figure. 3. Lithium mine, Salar del Hombre Muerto, Argentina. The brine in this salar is rich in lithium, and the mine concentrates the brine by pumping it into solar evaporation ponds. 2009 image from NASA’s EO-1 satellite.

Figure. 4. Salar de Uyuni, Bolivia.
Похожие работы
... 780 °C и выдерживали 18 часов на воздухе [6]. LiFePO4 получен аналогично, но в инертной атмосфере [10]. 1.3. Смешанные фторидофосфаты щелочных и переходных металлов Просмотр реферативных журналов, баз данных PDF-2 и ICSD обнаружил только три фазы формульного типа A+2MPO4F, из них с литием только одна: Li2NiPO4F [11]. Известны также Na2MnPO4F [12], Na2MgPO4F [13], Na4,6FeP2O8,6F0,4 [14, 15, ...
... веществом, как уже было сказано выше, являются только частые лекарственного патогенеза. Для большинства патогенезов лекарственных химических средств гомеопатия широко пользуется данными профессиональной токсикологии. II. Гомеопатические разведения (потенции) и способ их приготовления Лекарство, даваемое в ничтожно малом количестве, ...
... впервые получены следующие результаты: · Разработана обобщенная координационно-кластерная модель для описания взаимодействий и расчета термодинамических характеристик раствора неметалла в расплаве из трех металлических компонентов. · Установлена связь между термодинамическими свойствами (коэффициентами термодинамической активности и параметрами взаимодействия компонентов первого порядка) и ...
... слушает только THE RAINCOATS,а по большому счету "забил "на всю эту шумиху вокруг его творчества.На 12-ой церемонии вручения британских наград,проходившей 16 февраля в лондонском Alexandra Palase ,НИРВАНА получила титул "лучшей новой группы" в международной категории.Воспользовавшись паузой между студийными сейшенами,Крис Новоселич принял участие в аакции War Child в поддержку детей,пострадавших ...


0 комментариев