Навигация
6.1 Development of Lambda
Two Alternative Modes. After infecting the host, the lambda genome may start its replication; this results in the formation of multiple copies of the genome. The protein components necessary for the assembly of mature phage particles are synthesized by the coordinated expression of phage genes. Phage DNA is packaged inside a coat, and the mature phages are released into the environment after cell lysis. This mode of propagation is called the lytic cycle.
Alternatively, the phage genome may enter a dormant stage (prophage) by integrating itself into a bacterial genome by site-specific recombination; during this stage it is propagated along with the host in the subsequent progeny. This stage is termed lysogeny. Changes in environmental and physiological conditions may activate the prophage stage and trigger lytic events.
7. Phage Lambda as a vector
Figure 6. Bacteriophage
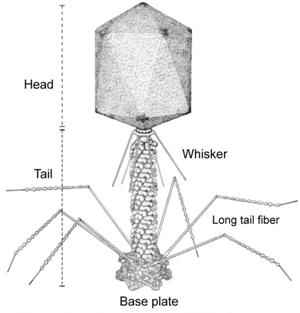
The large genome size and complex genetic organization of lambda had posed initial problems with its use as a vector. The problems, however, were surmounted through the sustained efforts of researchers, and lambda has been developed into an efficient vector.
The broad objectives in constructing various phage vectors are
- the presence of cloning sites only in the dispensable fragments,
- the capacity to accommodate foreign DNA fragments of various sizes,
- the presence of multiple cloning sites,
- an indication of incorporation of DNA fragments by a change in the plaque type,
- the ability to control transcription of a cloned fragment from promoters on the vector,
- the possibility of growing vectors and clones to high yield,
- easy and ready recovery of cloned DNA,
- introduction of features contributing to better biological containment.
There are several difficulties in the use of lambda as a vector.
Some of the problems and the general strategies adopted to overcome them are discussed in this section. Manipulation of Restriction Sites The major obstacle to the use of phage lambda as a cloning vector was essentially the presence of multiple recognition sites for a number of restriction enzymes in its genome.
Initially, all attempts were directed toward minimizing the number of EcoRI sites. Murray and Murray in 1974 were able to construct derivatives of lambda with only one or two EcoRI sites. Similarly, Rambach and Toillais constructed lambda derivatives with EcoRI sites only in the nonessential region of the genome by repeated transfer on restrictive and nonrestrictive hosts . After several cycles of digestion, packaging, and growth, phage derivatives with desirable restriction sites and full retention of infectivity were obtained. All but one HindIII sites were removed by recombination of known deletion mutants or substitutions. Recently, oligonucleotides with specific sequences have been synthesized and introduced into the bacteriophage lambda genome. This has provided a variety of cloning sites in the genome [5].
7.1 Size Limitation for Packaging
The second problem was the requirement of a minimum and maximum genome length (38 and 53 kbp, respectively) for the efficient packaging and for the production of viable phage particles. The viability of the bacteriophage decreases when its genome length is greater than 105% or less than 78% of that of wild-type lambda. Genetic studies of specialized transducing bacteriophages showed, however, that the central one-third of the genome, i.e., the region between the J and Ngenes, is not essential for lytic growth. The presence of a nonessential middle fragment of the phage genome was also revealed during construction of viable deletion mutants. These mutants lack most of the two central EcoRI B fragments which are not essential for lytic growth. However, too much DNA cannot be deleted because there is a minimum 38-kbp requirement essential for efficient packaging. The de novo insertion of DNA (even if heterogeneous) is essential for the formation of viable phages. This constitutes a positive selection for recombinant phages carrying insertions. This approach was successfully exploited in constructing recombinant phages carrying E. coli and Drosophila melanogaster DNA [8].
7.2 Transfection of Recombinant Molecules
The problem of transfection of recombinant molecules constructed in vitro was overcome by the successful in vitro assembly of viable and infectious phage particles. Two types of in vitro packaging systems have been developed so far, i.e., two-strain packaging and single-strain packaging.
Two-strain packaging.
The basis of the two-strain in vitrop ackaging system is the complementation of two amber mutations. Two lambda lysogens, each carrying a single amber mutation in a distinctly different gene, are induced and grown separately so that they can synthesize the necessary proteins. Neither of the lysogens alone is capable of packaging the phage DNA. The role of various phage products in DNA packaging has been studied in detail[3]. The E protein is the major component of the bacteriophage head, and in its absence all the viral capsid components accumulate. The D protein is involved in the coupled process of insertion of bacteriophage DNA into the prehead precursor and the subsequent maturation of the head. The A protein is required for the cleavage of the concatenated precursor DNA at the cos sites. Two phage lysogens carrying A and E or D and E mutations in the phage genome are induced separately, and cell extracts are prepared. Neither of the extracts can produce infectious phage particles. However, when the extracts are mixed, mature phage particles are produced by complementation.
The major drawback of the two-strain system is the competition of native phage DNA with recombinant molecules. In both the cell extracts, native phage DNA is also present and can be packaged with an efficiency equal to that of the chimeric DNA. This reduces the proportion of recombinants obtained in a library. The problem of regeneration of endogenous phages obtained in the library was partially overcome by the use of b2-deleted prophages, which poorly excise out of the host chromosome or by UV irradiation of packaging extracts.
Single-strain packaging.
Rosenberg have successfully developed a single-strain packaging system by introducing deletion in the cos region of prophage, rendering the prophage DNA unpackagable because cos is the packaging origin. Induction of the lysogen results in the intracellular accumulation of all protein components needed for packaging.
However, packaging of phage DNA is prevented by the lack of cos sites on the prophage DNA. On the other hand, exogenous DNA with cos sites is packaged efficiently to produce an infectious bacteriophage particle. The single-strain system is superior to two-strain system in having a lower background of parental phages. In addition, it uses E. coli C, which lacks the EcoK restriction system, as the host for the lysogen.
Похожие работы
... enable the seeds to survive the winter. Overwintering might allow the plant to become a weed or might intensify weedy properties it already possesses. Change in Herbicide Use Patterns Crops genetically engineered to be resistant to chemical herbicides are tightly linked to the use of particular chemical pesticides. Adoption of these crops could therefore lead to changes in the mix of chemical ...
... растений позволит значительно снизить её стоимость (Giddings et al., 2000). В заключение хотелось бы отметить, что несмотря на значительные достижения в области продукции реком-бинантных белков медицинского назначения в растениях, это направление находится лишь на начальном этапе своего развития. Учёные-биотехно-логи уверены, что в будущем рекомбинантные препараты, получаемые из генетически ...
0 комментариев